A few weeks ago, Lena Sophie Koch (NOCI- PhD student) discussed with us in her NOCI news item how close we can get to the human with a review on the gut-brain axis. This seems to be a burning question for many researchers of various organ-on-chip fields. So today I would like to share with you how close to the human we want to get and how a “Brain-on-a-chip” will help us to!
In the lab of Ype Elgersma at the Erasmus MC we are interested in neurodevelopmental disorders including Angelman Syndrome, Tuberous Sclerosis complex (TSC), Neurofibromatosis Type1 (NF1) and many others (click here for more information). For decades the only way to study brain function and malfunction was through the use of animal models. Recently new technologies, like human stem cell reprogramming and advanced cell culture methods, have paved the way to researching neurodevelopmental disorders in more relevant human brain models. However, verification of the biological and clinical relevance of these novel cell-based model systems remains challenging as human brain tissue is not readily available for comparative studies.
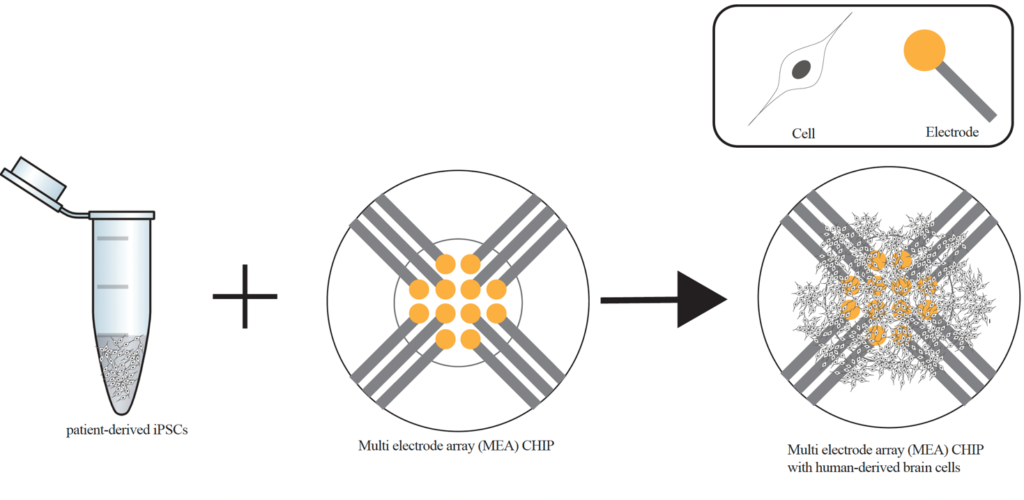
Currently, we are investigating how well we can model the clinical phenotype observed in genetic epilepsy patients in an in vitro experiment approach. Through collaboration with the clinicians at the Sophia Children’s hospital and the Erasmus MC, we can get in touch with patients suffering from mild to severe epilepsy. Patients can participate in the research by donating a blood sample. In our lab we can reprogram these blood cells into so-called induced pluripotent stem cells (iPSCs) and subsequently differentiate them into different types of brain cells. Since these patient-derived cells carry the genetic defect of the patient, they enable us to study how cellular and functional properties are affected in this patient.
Brain cells communicate with each other by small electrical signals, using small sensors attached to the patients scalp these electrical signals can be recorded. This technique, electroencephalography (EEG), is used to study brain function and diagnose epilepsy in humans.
You might still be wondering how we can model epilepsy ‘in a dish’, considering that the disease affects the whole brain. This is where high-tech brain-on-chip technology comes in place. So-called multi-electrode arrays (MEA) enable us to measure electrical currents and electrical activity within a cell culture dish. Combining this MEA-technology with iPSC differentiation provides us with a human in vitro model system for brain activity.
In practice this means that patient-derived cells are differentiated and grown on top of the electrodes of the MEA platform (see Figure). Within a few weeks these cells grow into highly connected networks of neurons and astrocytes. They communicate with each other via electrical signals, which can be measured by the electrodes of the MEA. With comparative studies between the electrical network patterns in patient-derived cells and healthy controls, we hope to be able to predict epilepsy severity and to gain better understanding on the epilepsy mechanism and brain pathology. Consequently, drug testing, severity prediction and eventually personalized medicine can be tailored for optimal efficacy in human using this novel in vitro model system.
Despite the vast complexity of the human brain, current technological advances enable us to develop relevant in vitro systems to step by step take us closer to model various human brain functions. For now, we are equipped with state-of-the-art tools and models. All that’s left to do is to learn how to put these tools to their best use. If you would like to know more about it, stay tuned for more updates on the NOCI news feed!
 Eva Niggl, NOCI PhD student at ErasmusMC
Eva Niggl, NOCI PhD student at ErasmusMC
