TECHNOLOGICAL CHALLENGES
The resulting knowledge, experimental tools and disease models will be integrated into three Technological Challenges covering the organ-on-chip process, specifically:
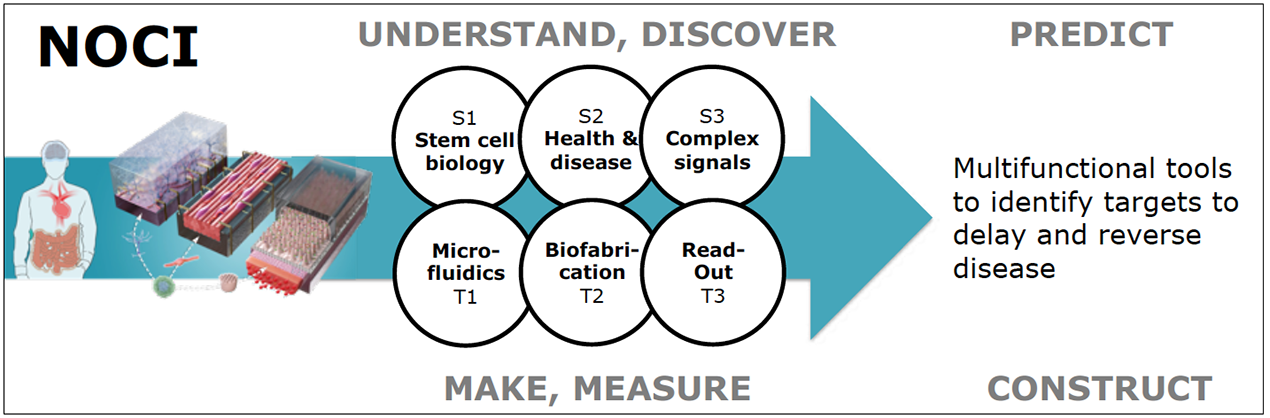
Chip design and production, including controlled biomechanics, biochemistry and fluid flow (T1)
Technological Challenge T1: Mastering microfluidic chip engineering with integrated sensors and actuators
Ten-year goal: To use advanced microengineering and nanotechnology to develop a robust microfluidic platform for multicellular co-culture with well-defined conditions of fluid flow, biomechanics and geometry, as well as by developing and integrating advanced biosensors and actuators.
Coordinating scientists: A. van den Berg (UT) and L. Sarro (TUD)
Collaborating partners: R. Dekker (Philips/TUD), M. Dogterom (TUD), D. Lohse, A. van der Meer, S. le Gac (UT), V. Orlova (LUMC), R. Passier (LUMC/UT)
Integration of stem cells and organoids (T2)
Technological Challenge T2: Mastering biofabrication of organs-on-chips using stem cells and organoids
Ten-year goal: The ability to engineer functional human tissues that capture the cellular niche include the microbiome and inflammatory components of the brain, intestine and vascular system suitable for multiplexing in organ-on-chip formats that are structured predominantly by controlled, hierarchical self-organization.
Coordinating scientists: C.L. Mummery (LUMC/UT) and H. Clevers (HI/UMCU)
Collaborating partners: V. Orlova (LUMC), A. van der Meer (UT), M. Netea (RU)
Relevant biomarkers as readout of the disease (T3)
Technological Challenge T3: Mastering functional and systems biological readout from organs-on-chips
Ten-year goal: Methods available to collect biomedically relevant samples from the multicellular co-cultures and process these with state-of-the-art high-throughput molecular biological tools. To ensure compatibility with existing data from conventional cell monocultures and clinical samples.
Coordinating scientists: C. Wijmenga (UMCG) and M. Ferrari (LUMC)
Collaborating partners: S. van Maarel, G. Terwindt, A. van den Maagdenberg (LUMC), J. Gribnau, S. Kushner, Y. Elgermsa (Erasmus MC), L. Sarro (TUD), I. Jonkers, L. Franke (UMCG), A. van den Berg, D. Lohse, L. Segerink, A. van der Meer (UT), R. Passier (LUMC/UT)
Through our existing links with the pharmaceutical sector (in part through hDMT), we will showcase the usability of complex human tissue models for screening new treatments and repurposing existing drugs. This is at the heart of human stem cell-, organoid- and organs-on-chip research in the Netherlands. We expect our program to impact Dutch biomedical research far beyond the diseases used as exemplars and the decade for which the work is planned.
Vision
- make realistic, multicellular and three-dimensional organ-on-chip models of any person each with its unique genetic background, of any tissue of the adult body (minimally the gut, heart and regions of the brain) and that these show normal hormonal and cytokine responses and expected disease features.
- measure how cells and tissues in these models function in health and disease and interact with exogenous factors, capturing genetic and epigenetic and environment interactions and individual cellular and molecular responses.
- understand disease predisposition and progression, and identify factors that can prevent or revert the phenotype
- discover new targets for novel treatments and biomarkers and metabolites for disease diagnosis and monitoring disease onset, progression and therapeutic response.
- predict disease predisposition and severity, which individuals will develop the disease and which microbes exacerbate or reduce the disease state.
- construct multicellular mass-produced chips, which can be linked to mimic communication between organs through metabolites or other cytokines produced, and body-on-chip devices are feasible.