X chromosome inactivation and X-linked intellectual disability
Already 8 months ago, I started my PhD in the Gontan lab at de Department of Developmental Biology at the Erasmus MC. Before starting my PhD, I studied neurobiology and performed research projects on for example Alzheimer’s disease in mouse models and autism spectrum disorder in human induced pluripotent stem cells (hiPSCs). These experiences confirmed that I really wanted to pursue a career in neurobiology. In my current PhD project I am even able to combine my interest for neuroscience with a fascinating new topic: X chromosome inactivation.
Sex chromosomes are the only chromosomes that differ in their number of copies between male and female cells: male cells contain 1 X and 1 Y sex chromosome, while female cells comprise 2 X chromosomes. The Y chromosome is significantly smaller than other chromosomes, encoding only around 70 protein-coding genes, whereas the X chromosome encodes around 800 protein-coding genes. As a result, there is random inactivation of 1 X chromosome during early female development to achieve dosage compensation between both sexes.
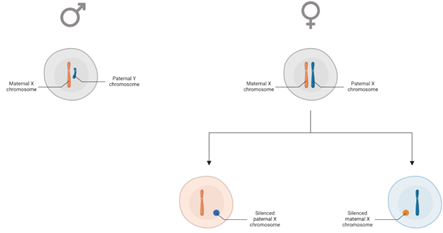
Random X chromosome inactivation occurs during early female development. As a result, males contain a homogenous population of cells expressing the maternal X chromosome, with females are typically a mosaic of cells expressing the maternal or paternal X chromosome.
X chromosome inactivation (XCI) is initiated upon embryonic implantation by upregulation and spreading of the long non-coding RNA XIST along the future inactive X chromosome. In mice, an important activator of XIST and thus XCI is the protein RNF12. As the mechanism of action of RNF12 in humans is still unknown, the first part of my project focuses on elucidating the role of RNF12 in human XCI. In addition to its role in XCI in females, pathogenic variants of RNF12 in males have been shown to cause the neurodevelopmental disorder Tonne-Kalscheuer syndrome (TOKAS), defined as a syndromic form of X-linked intellectual disability (XLID). Heterozygous female carriers of the pathogenic RNF12 variants do not display symptoms, but, instead of random XCI, they display extreme skewing of XCI, indicating that the vast majority of cells inactivate the X chromosome with the mutant RNF12 allele. The second part of my project will focus on elucidating the mechanism by which RNF12 mutations induce TOKAS in male patients. For both research lines, I will use human induced pluripotent stem cells (hiPSCs), which are stem cells generated by reprogramming human fibroblasts. The hiPSCs will also be differentiated into cortical neuronal networks, which will hopefully give us insight into the effect on RNF12 on human neurodevelopment. The neuronal networks will be analyzed using multi-electrode arrays, which enables us to investigate their electrophysiological properties and compare these between control and RNF12 mutant lines.
I think it’s absolutely fascinating how the difference in sex chromosomes between males and females leads to sex bias in disease and specifically in neurodevelopmental disorders. Therefore, I’m very excited to contribute to the research in this field. Although I’m still in the beginning of my PhD trajectory, I’ve already experienced the value of being part of NOCI. I’ve already had the chance to attend three NOCI practical training and retreats and this network already resulted in valuable feedback and collaborations.

Kyra Swildens
PhD candidate at EMC
