Two years ago, I started my PhD in the Immunogenetics lab run by Sebo Withoff and Iris Jonkers at the Department of Genetics of the University Medical Center Groningen in the context of the NOW Gravitation program ‘Netherlands Organ-on-Chip Initiative’ (NOCI). In our group we work with organoids and organ-on-chip systems as models for the intestine and the liver. My project mainly focusses on the intestine-on-chip, which we use to study coeliac disease pathophysiology.
Coeliac disease, also known as gluten intolerance, is a multifactorial complex disease where gluten intake triggers inflammatory responses in the small intestines of genetically predisposed individuals, in ~1% of the Western population. Modelling the complexity of this coeliac disease, including genetic, environmental (e.g. gluten; the microbiome) and immunological susceptibility factors is very challenging. Most currently used model systems do not contain the genetic susceptibility nor the complexity of the disease, specifically the combination of tissues involved. To overcome these shortcomings, we (NOCI PhD’s Renée Moerkens and Joram Mooiweer) have established intestinal organoid and intestine-on-chip platforms using the commercially available Emulate system, based on induced pluripotent stem cells (iPSCs) or adult stem cells (ASCs) cells obtained from patients and control individuals [1, 2].
In my project I compare intestinal epithelial cells (IECs) either derived from iPSCs (iPSC-IECs) or from ASCs (ASC-IECs). While iPSC-IECs are more fetal and are not derived from the intestine itself, ASC-IECs are thought to preserve epigenetic signatures from their original environment [3]. Utilizing both iPSC-IECs and ASC-IECs from patients allows to explore the role of (epi)genetic factors in barrier functionality in the context of celiac disease. At the moment, I am differentiating iPSCs towards iPSC-IECs and optimizing the systems to grow both iPSC-IECs and ASC-IECs in similar culture conditions. Multiple mature intestinal cell-types can be found in our iPSC-IEC chips, which self-organize in fold-like structures, creating a tight barrier (Figure 1A) [1]. Simultaneously we are piloting with ASC-IECs on our chips to characterize the cell-types on these barriers (Figure 1B).

Eline Smits, PhD candidate at UMCG
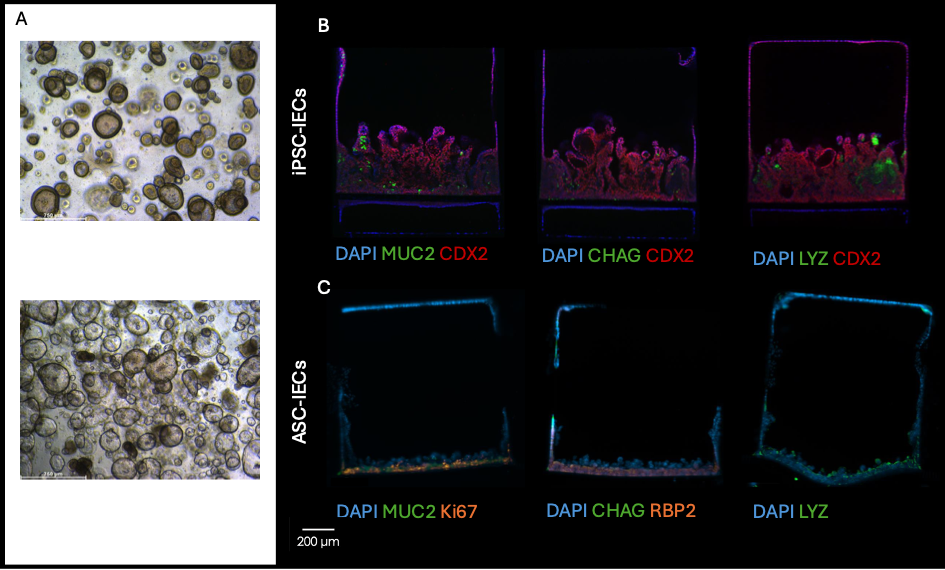
Intestinal epithelial cells derived from induced Pluripotent stem cells (iPSCs) and adult stem cells (ASCs) grown as organoids and on the Emulate chip. A) human intestinal organoid derived from iPSC (top) and ASC (bottom). B) Depicted are some results from Moerkens et al. [1]. These iPSC-IECS grown on EM-DM show mature cell types, like goblets cells (MUC2), enteroendocrine cells (CHAG) and paneth-like cells (LYZ). These images are made with confocal imaging. C) Preliminary pilot data from ASC-IECs grown on our chips. Cells are stained for proliferation (Ki67), intestinal marker (CDX2) or enterocyte marker (RBP2). Nuclei stained with DAPI. These images are made with a fluorescence microscope.
I aim to compare these systems (ASC-IECs and iPSC-IECs) and want to characterize the effect of their epigenetic status on barrier function. The barrier will be characterized by confocal imaging, FITC-dextran permeability assays and (single cell) RNA- and ATAC-sequencing. Additionally, barriers will be exposed to gluten peptides to elucidate reactivity towards the peptides and gluten transport over the barrier.
Being part of the Gravitation program NOCI is a unique and rewarding experience. A few months after the start of my PhD I had the opportunity to join my first NOCI consortium meeting, where I got introduced to the welcoming group of scientists. I loved the open atmosphere created by the PhD-students, post-docs and group leaders. Besides these annual consortium retreats we benefit from an exciting training program for the junior scientists of NOCI, including retreats and trainings to develop our skills even further.
References:
Moerkens R, Mooiweer J, Ramírez-Sánchez AD, et al. An iPSC-derived small intestine-on-chip with self-organizing epithelial, mesenchymal, and neural cells. Cell Rep. 2024;43(7):114247. doi:10.1016/j.celrep.2024.114247
Moerkens R, Mooiweer J, Smits E, et al. Intestine-on-chip enhances nutrient and drug metabolism and maturation of iPSC-derived intestinal epithelial cells relative to organoids and Transwells. BioRxiv. Published online July 01, 2024. doi: doi.org/10.1101/2024.06.30.601390
Simpson HL, Smits E, Moerkens R, et al. Human organoids and organ-on-chips in coeliac disease research. Trends Mol Med. Published online October 23, 2024. doi:10.1016/j.molmed.2024.10.003
