When I was an undergraduate student, I learned in a journal club that the lab of Hans Clevers in Utrecht had just shifted the boundaries of cell biology and tissue engineering: Single adult stem cells from patients’ biopsies – cultured under the right conditions – would give rise to mini-organs (termed “organoids”) that recapitulate the entire repertoire of epithelial cell types of the human gut. With one frontier falling, countless new opportunities arose, just waiting to be seized. One such question was of particular interest to me. Can we re-build the microenvironment – consisting of diverse immune and stromal cells as well as bacteria – around organoids to understand their interactions in cancer development?
Upon joining the Clevers lab in 2017 for my PhD, two things became clear quite quickly. First, that such a big biological question needs specialized model systems and techniques to be answered. Second, that creating these models would require a multi-disciplinary approach in which we use our understanding of cell biology for engineering the microenvironment bottom-up.
 I was in luck, since in 2018 exactly this opportunity arose: I could join the Netherlands Organ on a Chip Initiative (NOCI), which was founded to bring together engineers and cell biologists to create new organ-on-a-chip models. Four years of NOCI meant a steep learning curve for me on engineering approaches around microfluidic devices, in depth theoretical and practical workshops on all aspects of organ-on-a-chip technology and an unparalleled team spirit among the group of NOCI trainees. The shared interest in gut models and host-microbe interactions with the group of Sebo Withoff led to extensive laboratory visits, frequent discussions and collaborations between the Groningen and Utrecht sites.
I was in luck, since in 2018 exactly this opportunity arose: I could join the Netherlands Organ on a Chip Initiative (NOCI), which was founded to bring together engineers and cell biologists to create new organ-on-a-chip models. Four years of NOCI meant a steep learning curve for me on engineering approaches around microfluidic devices, in depth theoretical and practical workshops on all aspects of organ-on-a-chip technology and an unparalleled team spirit among the group of NOCI trainees. The shared interest in gut models and host-microbe interactions with the group of Sebo Withoff led to extensive laboratory visits, frequent discussions and collaborations between the Groningen and Utrecht sites.
Beyond these specific interactions, the time in the NOCI made clear to me how many frontiers in organoid technology may be overcome using organ-on-a-chip approaches. By populating microfluidic devices with organoid-derived cells, we can create versatile organs-on-chips that increase the control over many parameters such as shape and luminal flow. Using the many insights on media compositions and differentiation trajectories from almost 15 years of organoid research, we can unite the best of organoids and organs-on-chips to create highly representative human in vitro models.
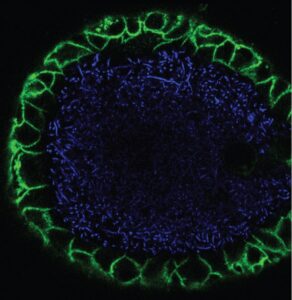 As part of the NOCI consortium and many collaborations, my partner in (detecting microbial) crime Cayetano Pleguezuelos-Manzano and I improved organoid-bacteria co-culture techniques (https://www.nature.com/articles/s41596-021-00589-z) and derived insights into the roles of specific gut bacteria in inducing mutations in colorectal cancer (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8142898/, together with Axel Rosendahl-Huber and Ruben van Boxtel). Our work is just a small section of a rapidly evolving field around host-microbe co-cultures on organs-on-chips that we have recently reviewed (https://www.cell.com/cell-host-microbe/fulltext/S1931-3128(21)00150-5).
As part of the NOCI consortium and many collaborations, my partner in (detecting microbial) crime Cayetano Pleguezuelos-Manzano and I improved organoid-bacteria co-culture techniques (https://www.nature.com/articles/s41596-021-00589-z) and derived insights into the roles of specific gut bacteria in inducing mutations in colorectal cancer (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8142898/, together with Axel Rosendahl-Huber and Ruben van Boxtel). Our work is just a small section of a rapidly evolving field around host-microbe co-cultures on organs-on-chips that we have recently reviewed (https://www.cell.com/cell-host-microbe/fulltext/S1931-3128(21)00150-5).
Beyond these efforts, a fantastic team from the Clevers lab around Cayetano Pleguezuelos-Manzano, Adriana Marinez-Silgado and Sangho Lim is pushing new boundaries in host-microbe and epithelium-immune interactions through collaborations within the NOCI consortium. For myself, the training and collaboration within the NOCI group has been an invaluable opportunity to see many aspects of organ-on-a-chip-based science. All these experiences have led me to start a research group in the Microbiome and Cancer division of the German Cancer Research Center in Heidelberg with a focus exactly where so many new frontiers lie: At the intersection of organoid and organ-on-a-chip approaches.

Jens Puschhof
Former NOCI PhD-student at Hubrecht Institute
