As a neurobiologist, I have been intrigued by the brain since my study Medical Biology in Utrecht. It is the most mysterious organ of the body, containing our thoughts, dreams and our curiosity, with much left to discover how it functions and how it’s dysfunction can cause devastating diseases. After a PhD at the Dutch Brain Institute in Amsterdam studying Alzheimer’s disease and a postdoc period on Fragile X Syndrome, I landed at the Psychiatry Department of Erasmus MC where I now supervise a talented team working on stem cell models of neuropsychiatric disorders within the lab of Professor Steven Kushner. My focus has always been on building cellular models of the brain and using them for studying the neurobiological underpinnings of brain diseases.
In this ongoing puzzle, induced pluripotent stem cell (iPSC) technology has revolutionized the field. The main reason why the brain is still so mysterious to this day, is largely due to its inaccessibility. Since the emergence of iPSC technology we finally have the opportunity to study living brain cells in a dish derived directly from patients. We have used this technology, as many others, to develop standardized neural network models and used them to functionally compare brain cells from patients with those from healthy individuals, for instance in our family-based schizophrenia study. Other potential applications are for instance drug testing, like we did for a drug that caused serious brain pathology and even a death in a French drug trial. Working together with our lead collaborators on the story in Leiden, we found that this drug was toxic to human neurons, while it seemed harmless in rodent neurons during preclinical testing, emphasizing the need for testing therapeutics in human cells. Another example is a large collateral project that just started, together with NOCI partner Ype Elgersma and collaborators from Nijmegen, Utrecht and Leiden. In this PSIDER project, we aim towards therapeutic discovery for neurodevelopmental disorders using a human iPSC-based neuronal platform. In another project from ZonMw, Create2Solve, we are building on our animal-free adherent cortical organoid platform (see Figure 1) to implement myelination and inflammation studies together with data analytics company Core Life Analytics .
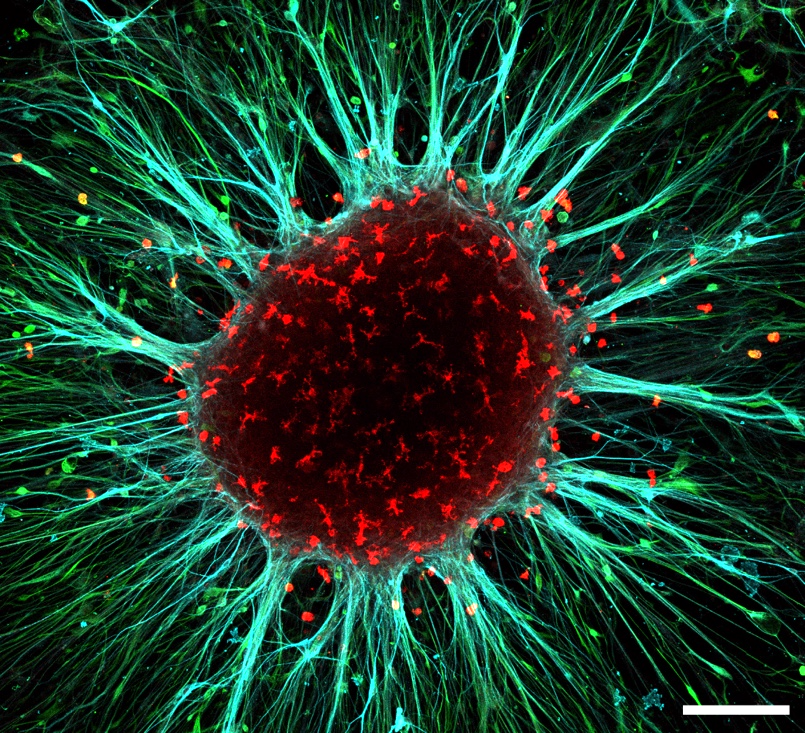
Figure 1. Adherent cortical organoid after 150 days in culture showing neurons (MAP2 staining in blue), astrocytes (S100b staining in green) and microglia (IBA1 staining in red). Scalebar 200mm. Image by Sakshi Bansal and Mark van der Kroeg.
The Netherlands Organ on Chip Initiative (NOCI) provides a great network to share expertise and important multidisciplinary collaborations. Especially for the brain, this is such an exciting time to combine stem cell-derived brain modeling with the engineering expertise from the technical NOCI partners, keeping up with the development rate of different models, ranging from a diversity of brain cell types in 2D to 3D organoids and assembloids combining different brain areas.
For these multidisciplinary collaborations, we needed to learn to speak each other’s language. At the start of NOCI, I had never heard of silicon wafers and PDMS stamps and I think the engineers had to get used to human neurons derived from iPSCs that are not as easy-going as immortalized cell lines. Now we see ongoing collaborations strengthen across different disciplines, including interdisciplinary students. In our group, PhD student Maurits Unkel just joined the NOCI task force. With his bioinformatics background he is a perfect addition to the team for handling complex data that arise from advanced technologies such as multi electrode arrays (MEAs) and single cell RNA sequencing.
We look forward to continued collaborations with our NOCI partners. For us biologists, being able to design cell culture wells with different geometries is already super exciting and plans for integrating 3D pillar electrodes in our brain organoid models give amazing future perspectives.
With all the expertise that comes together in NOCI, I think there are great times ahead, together creating tools to understand human biology and ultimately combining multiple organ models on microfluidic chips aimed towards personalized medicine.

Femke de Vrij, Associate Professor
NOCI researcher at Erasmus MC
